TPI 287: Late Stage, Novel Blood Brain Barrier Permeable Abeotaxane for Treatment of Brain Malignancies
About TPI 287
TPI 287 is a small molecule belonging to the taxane diterpenoid (taxoid) family, specifically to the abeotaxane class. Originally isolated from the bark of the Yew tree, TPI 287 is a novel semi-synthetic microtubule inhibitor tested in over 350 patients to date.
In clinical trials, TPI 287 has been administered as monotherapy as well as in combination with temozolomide and bevacizumab for the treatment of recurrent glioblastoma, neuroblastoma, medulloblastoma, refractory prostate cancer and in tauopathy disease (dementia caused by aggregation of abnormal tau proteins in the brain). Like other taxanes, TPI 287 inhibits cancer cell growth by disrupting microtubule dynamics which are required for cell division. However, unlike all other known taxanes, TPI 287 is not a substrate for a wide spectrum of efflux pumps that constitute the blood-brain barrier (BBB), including PgP, which allows TPI 287 to reach the brain in high concentrations.
Program Highlights
- Evaluated in multiple Phase 1 and Phase 2 studies
- Engaging with regulators to advance into registrational study
- Orphan Drug Designation in multiple indications with 7 years market exclusivity in the U.S.
Compelling GBM Response and Overall Survival
Significant Reduction in GBM Tumor Volume
Improved GBM Survival In Combination with Bevacizumab
Taxanes
Taxanes are cytotoxic chemotherapeutic agents that have been used clinically for many decades. Like anthracyclines, they are a trusted class of first-line chemotherapy drugs. Taxanes primarily function by disrupting microtubule function. Microtubules are polymers of tubulin that form part of the cytoskeleton and provide structure and shape to cells and help to move cellular components throughout the cell and are thus essential to the process of mitosis, that part of the cell life cycle when replicated chromosomes split apart to form two new nuclei. Cancer cells are more sensitive to inhibition of mitosis than normal cells because they are growing via continuous division.
Historically, taxanes have not been used in neuro-oncology applications because as a class they lack the ability to penetrate the BBB. Since TPI 287 is not a substrate for any of the important BBB efflux pumps, it can concentrate in the brain, which has been demonstrated by several preclinical studies. Once inside the brain, TPI 287 can then access tumor cells and function much like other taxanes in obstructing the key functional dynamic associated with mitosis - stabilizing GDP-bound tubulin in the microtubules thus inhibiting the process of cell division by preventing the depolymerization or shortening of the microtubules.
Clinical Studies
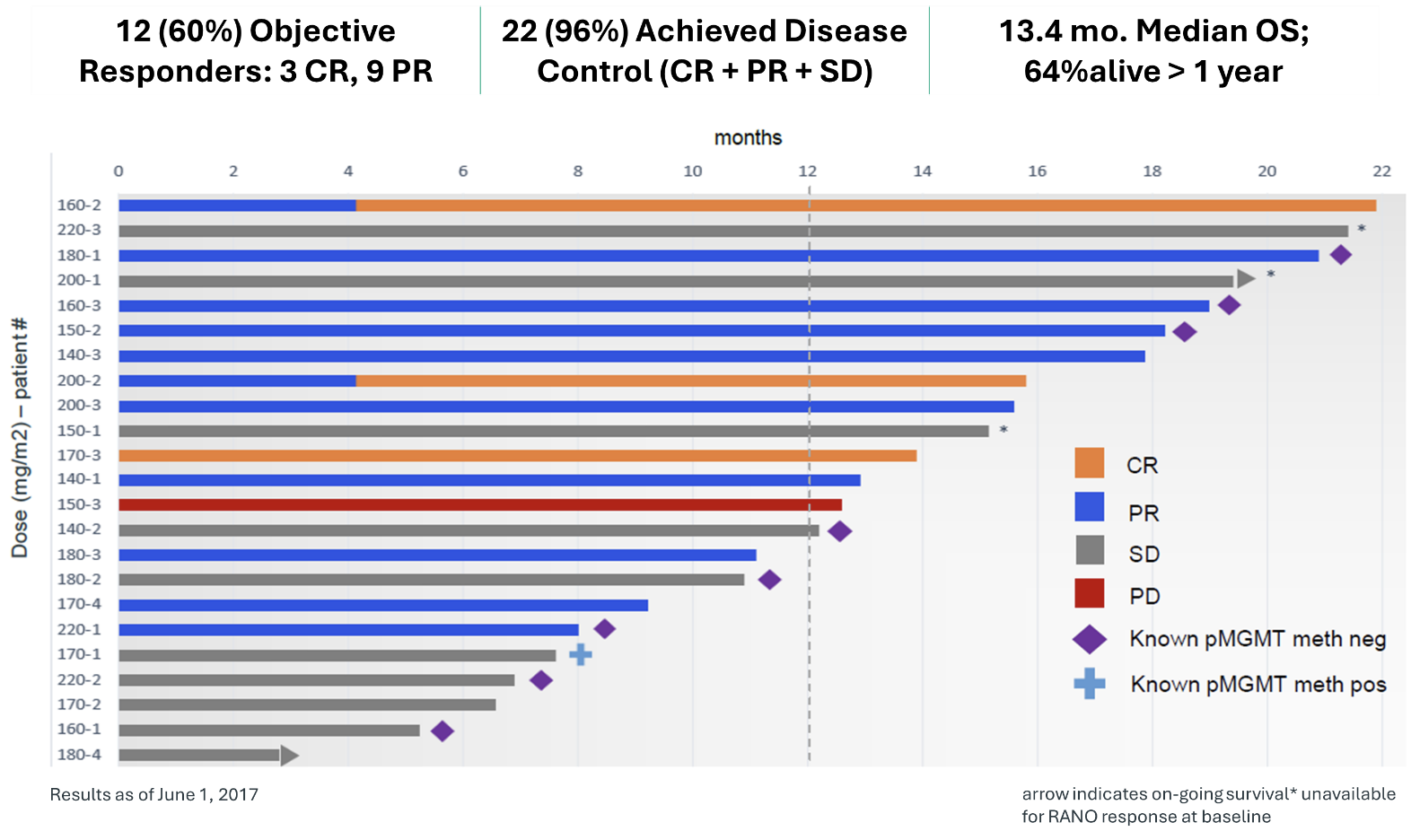
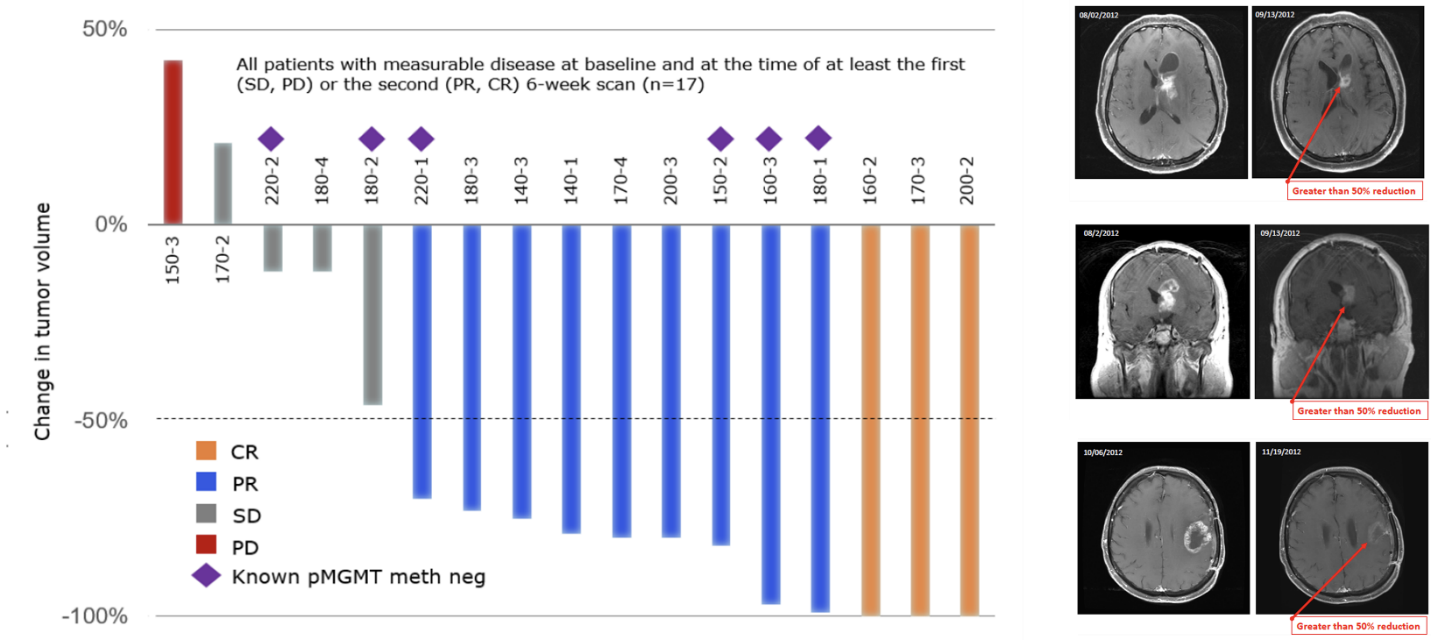
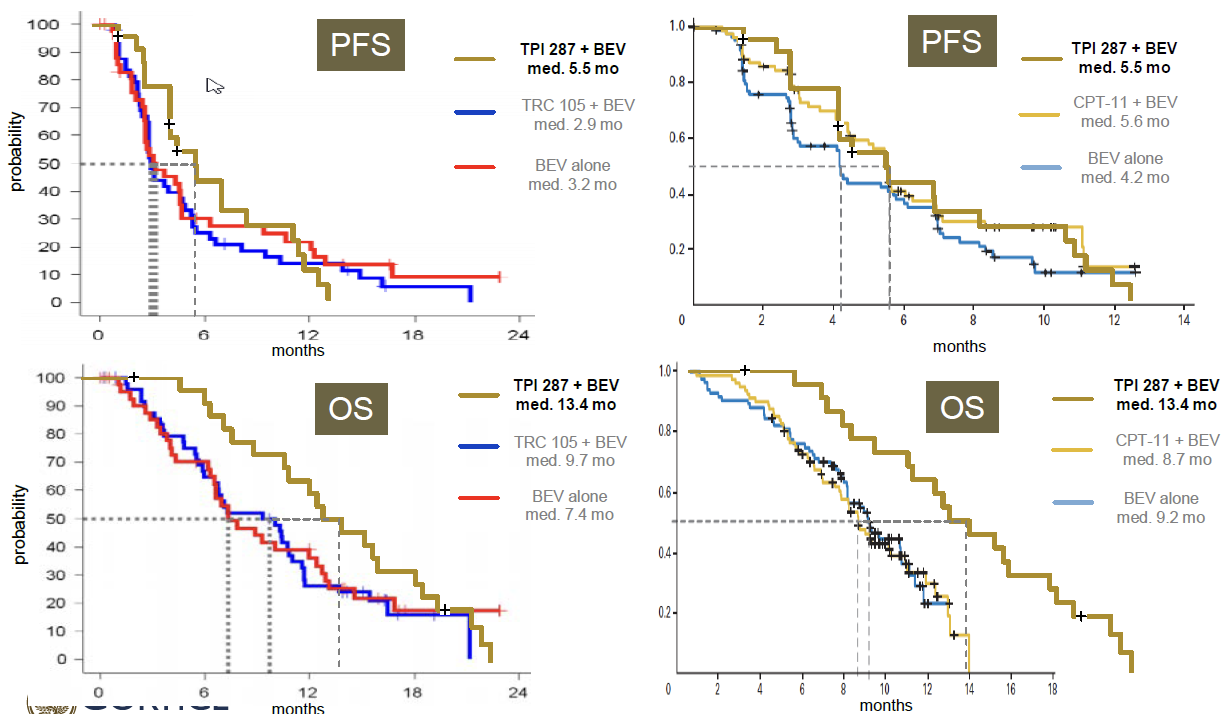
Between 2013 and 2015 TPI 287 was tested in combination with bevacizumab in 24 patients with recurrent glioblastoma multiforme (GBM) with up to two relapses after prior therapy at six clinical centers. TPI 287 was administered intravenously once every 3 weeks in a 22 day cycle, and the dose was escalated to evaluate dose-limiting toxicities (DLT) and the maximum tolerated dose (MTD). Safety was the primary endpoint of this study. There were no dose limiting toxicities observed and the safety profile was consistent with other microtubule-active drugs as well as with bevacizumab alone. Results demonstrated an objective response rate (ORR) of 60% and a disease control rate (DCR) of 96% (all subjects with responses and stable disease) in 23/24 subjects available for evaluation. Progression-free survival (PFS) of 5.5 months and overall survival (OS) of 13.4 months compare favorably to therapy with earlier bevacizumab studies, either as monotherapy or in combination. In similar patients, these studies showed a PFS of between 3 and 4 months and an OS between 7 and 9 months. The data from this study were recently published in a manuscript titled, “Phase 1 trial of TPI 287, a microtubule stabilizing agent, in combination with bevacizumab in adults with recurrent glioblastoma1,” in Neuro-Oncology Advances.
Publications and Presentations
References
- 1. Neuro-Oncology Advances, Volume 6, Issue 1, January-December 2024, vdae009, https://doi.org/10.1093/noajnl/vdae009